IONIC LIQUIDS AS POTENTIAL CO-CATALYST FOR CO2 ELECTROCHEMICAL REDUCTION
DOI:
https://doi.org/10.22452/mjs.vol43sp1.9Keywords:
Ionic liquids, CO2 conversion, electrochemical reduction, COSMO-RS, TurbomoleAbstract
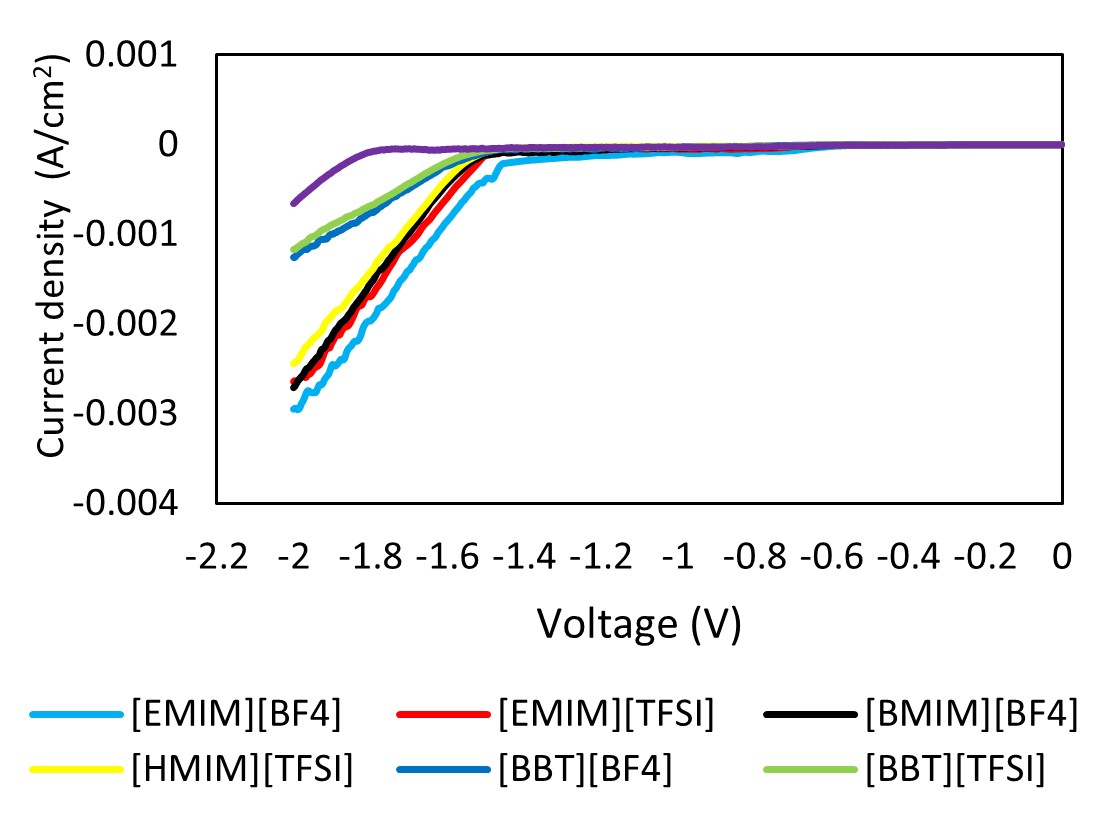
Carbon dioxide electrochemical reduction (CO2ER) presents numerous advantages in mitigating greenhouse gas emissions by converting CO2 into value-added chemicals and can be integrated with renewable energy sources such as solar and wind. Nevertheless, establishing an electrochemically stable catalytic system that can effectively decrease the overpotential while maintaining high current density and faradaic efficiency is challenging. The precise mechanisms causing the reactions and the specific functions of the electrode with electrolytes are still not fully understood. Hence, a significant increase in attention has been paid to using ionic liquids (ILs) as electrolytes for CO2ER. This phenomenon is attributed to the unique capabilities of ILs to reduce overpotential, increase current density, and improve electrochemical stability. Therefore, this study evaluated the effect of incorporating ILs into electrolytes to comprehend the cation and anion influences on CO2ER reactions. Linear sweep voltammetry (LSV) and chronoamperometry (CA) were employed to examine the reduction peaks and current density values for different electrolytes, respectively. Consequently, a 0.1 M NBu4PF6 acetonitrile solution containing 1-ethyl-3-methylimidazolium tetrafluoroborate [EMIM][BF4] demonstrated a significantly lower onset potential of the reduction by 320 mV. A reduced CO2ER efficiency involving ILs with long alkyl chains was also observed. Meanwhile, a novel hypothesis concerning molecular orbitals for the CO2ER reaction mechanism was discussed. Overall, various performance variables (reduction stability, applied potential, and current density) of CO2ER were improved using cations with short alkyl chains, anions with high highest occupied molecular orbital (HOMO) levels, and appropriate solvation media. These findings can serve as selection criteria to aid in choosing appropriate ionic liquids for CO2 electrochemical reduction (CO2ER).
Downloads
References
Aki, S. N., Mellein, B. R., Saurer, E. M., & Brennecke, J. F. (2004). High-pressure phase behavior of carbon dioxide with imidazolium-based ionic liquids. The Journal of Physical Chemistry B, 108(52), 20355-20365.
Azra, N., Nazir, F., Roosh, M., Khalid, M. A., Mansoor, M. A., Khan, S. B., & Iqbal, M. (2022). Extraction of Pb (II) and Co (II) using N, N-dioctylsuccinamate based room temperature ionic liquids containing aliphatic and aromatic cations. Arabian Journal of Chemistry, 15(9), 104099.
Bi, Z.x., Guo, R.t., Hu, X., Wang, J., Chen, X., & Pan, W.g. (2022). Research progress on photocatalytic reduction of CO2 based on LDH materials. Nanoscale, 14(9), 3367-3386.
Carlesi, C., Carvajal, D., Vasquez, D., & Arratia, R. S. (2014). Analysis of carbon dioxide-to-methanol direct electrochemical conversion mediated by an ionic liquid. Chemical Engineering Processing: Process Intensification 85, 48-56.
Every, H. A., Bishop, A. G., MacFarlane, D. R., Orädd, G., & Forsyth, M. (2004). Transport properties in a family of dialkylimidazolium ionic liquids. Physical Chemistry Chemical Physics, 6(8), 1758-1765.
Friedlingstein, P., Andrew, R. M., Rogelj, J., Peters, G. P., Canadell, J. G., Knutti, R., . van Vuuren, D. P. (2014). Persistent growth of CO2 emissions and implications for reaching climate targets. Nature geoscience, 7(10), 709-715.
Fukui, K. (1975). Theory of Orientation: Springer.
Gu, J., Liu, S., Ni, W., Ren, W., Haussener, S., & Hu, X. (2022). Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nature Catalysis, 5(4), 268-276.
Hori, Y., Murata, A., Kikuchi, K., & Suzuki, S. (1987). Electrochemical reduction of carbon dioxides to carbon monoxide at a gold electrode in aqueous potassium hydrogen carbonate. Journal of the Chemical Society, Chemical Communications, 728-729.
Hori, Y., Wakebe, H., Tsukamoto, T., & Koga, O. (1994). Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochimica Acta, 39(11-12), 1833-1839.
Hori, Y.,(2008). Electrochemical CO2 reduction on metal electrodes. In Modern aspects of electrochemistry (pp. 89-189): Springer.
Khan, H. W., Khan, M. K., Moniruzzaman, M., Al Mesfer, M. K., Danish, M., Irshad, K., . Chelliapan, S. (2023). Evaluating ionic liquids for its potential as eco-friendly solvents for naproxen removal from water sources using COSMO-RS: Computational and experimental validation. Environmental Research, 116058.
Khan, H. W., Reddy, A. V. B., Nasef, M. M. E., Bustam, M. A., Goto, M., & Moniruzzaman, M. (2020). Screening of ionic liquids for the extraction of biologically active compounds using emulsion liquid membrane: COSMO-RS prediction and experiments. J. Mol. Liq., 309, 113122.
Klamt, A. (2005). COSMO-RS: from quantum chemistry to fluid phase thermodynamics and drug design. Germany: Elsevier.
Klamt, A., Jonas, V., Bürger, T., & Lohrenz, J. C. (1998). Refinement and parametrization of COSMO-RS. J. Phys. Chem A, 102(26), 5074-5085.
Lau, G. P., Schreier, M., Vasilyev, D., Scopelliti, R., Grätzel, M., & Dyson, P. J. (2016). New insights into the role of imidazolium-based promoters for the electroreduction of CO2 on a silver electrode. Journal of the American Chemical Society, 138(25), 7820-7823.
Lau, G. P. S., Schreier, M., Vasilyev, D., Scopelliti, R., Grätzel, M., & Dyson, P. J. (2016). New Insights into the Role of Imidazolium-Based Promoters for the Electroreduction of CO2 on a Silver Electrode. Journal of the American Chemical Society, 138, 7820-7823.
Liu, C., Li, Y., Takao, M., Toyao, T., Maeno, Z., Kamachi, T., . . . Shimizu, K.i. (2020). Frontier molecular orbital based analysis of solid–adsorbate interactions over group 13 metal oxide surfaces. The Journal of Physical Chemistry C 124(28), 15355-15365.
Mohammed, S. A. S., Yahya, W. Z. N., Bustam, M. A., & Kibria, M. G. (2023). Experimental and Computational Evaluation of 1, 2, 4-Triazolium-Based Ionic Liquids for Carbon Dioxide Capture. Separations, 10(3), 192.
Mohammed, S. A. S., Yahya, W. Z. N., Bustam, M. A., Kibria, M. G., Masri, A. N., & Kamonwel, N. D. M. (2022). Study of the ionic liquids’ electrochemical reduction using experimental and computational methods. Journal of Molecular Liquids, 119219.
Monteiro, M. C., Dattila, F., Hagedoorn, B., García-Muelas, R., López, N., & Koper, M. T. (2021). Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nature Catalysis, 4(8), 654-662.
Navarra, M., Abbati, C., & Scrosati, B. (2008). Properties and fuel cell performance of a Nafion-based, sulfated zirconia-added, composite membrane. Journal of power sources, 183(1), 109-113.
Neyrizi, S., Kiewiet, J., Hempenius, M. A., & Mul, G. (2022). What It Takes for Imidazolium Cations to Promote Electrochemical Reduction of CO2. ACS Energy Letters, 7(10), 3439-3446.
Rosen, B. A., Haan, J. L., Mukherjee, P., Braunschweig, B., Zhu, W., Salehi-Khojin, A., . Masel, R. I. (2012). In situ spectroscopic examination of a low overpotential pathway for carbon dioxide conversion to carbon monoxide. Journal of Physical Chemistry C, 116, 15307-15312.
Rosen, B. A., Salehi-Khojin, A., Thorson, M. R., Zhu, W., Whipple, D. T., Kenis, P. J. A., & Masel, R. I. (2011). Ionic liquid-mediated selective conversion of CO2 to CO at low overpotentials. Science, 334, 643-644.
Shi, Z., Yang, H., Gao, P., Chen, X., Liu, H., Zhong, L., . Sun, Y. (2018). Effect of alkali metals on the performance of CoCu/TiO2 catalysts for CO2 hydrogenation to long-chain hydrocarbons. Chinese Journal of Catalysis, 39(8), 1294-1302.
Snuffin, L. L., Whaley, L. W., & Yu, L. (2011). Catalytic electrochemical reduction of CO2 in ionic liquid EMIMBF3Cl. Journal of The Electrochemical Society, 158, 155-158.
Tian, J., Yu, L., Xue, R., Zhuang, S., & Shan, Y. (2022). Global low-carbon energy transition in the post-COVID-19 era. Applied energy 307, 118205.
Ueno, K., Tokuda, H., & Watanabe, M. (2010). Ionicity in ionic liquids: correlation with ionic structure and physicochemical properties. Physical Chemistry Chemical Physics, 12(8), 1649-1658.
Vasilyev, D. V., Shyshkanov, S., Shirzadi, E., Katsyuba, S. A., Nazeeruddin, M. K., & Dyson, P. J. (2020). Principal descriptors of ionic liquid co-catalysts for the electrochemical reduction of CO2. ACS Applied Energy Materials, 3(5), 4690-4698.
Whipple, D. T., Finke, E. C., & Kenis, P. J. (2010). Microfluidic reactor for the electrochemical reduction of carbon dioxide: the effect of pH. Electrochemical Solid-State Letters, 13(9), B109.
Wojeicchowski, J. P., Abranches, D. O., Ferreira, A. M., Mafra, M. R., Coutinho, J. o. A., & Engineering. (2021). Using COSMO-RS to Predict Solvatochromic Parameters for Deep Eutectic Solvents. ACS Sustainable Chemistry, 9(30), 10240-10249.
Yang, H., Xu, Z., Fan, M., Gupta, R., Slimane, R. B., Bland, A. E., & Wright, I. (2008). Progress in carbon dioxide separation and capture: A review. Journal of environmental sciences, 20(1), 14-27.
Downloads
Published
How to Cite
Issue
Section
License
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).





