CHARACTERISATION OF EGG WHITE-IMPREGNATED ACTIVATED CARBON FOR CO2 ADSORPTION APPLICATION
DOI:
https://doi.org/10.22452/mjs.vol43sp1.4Keywords:
Activated carbon, CO2 capture, CO2 adsorption, Natural Amino Acids, Egg WhiteAbstract
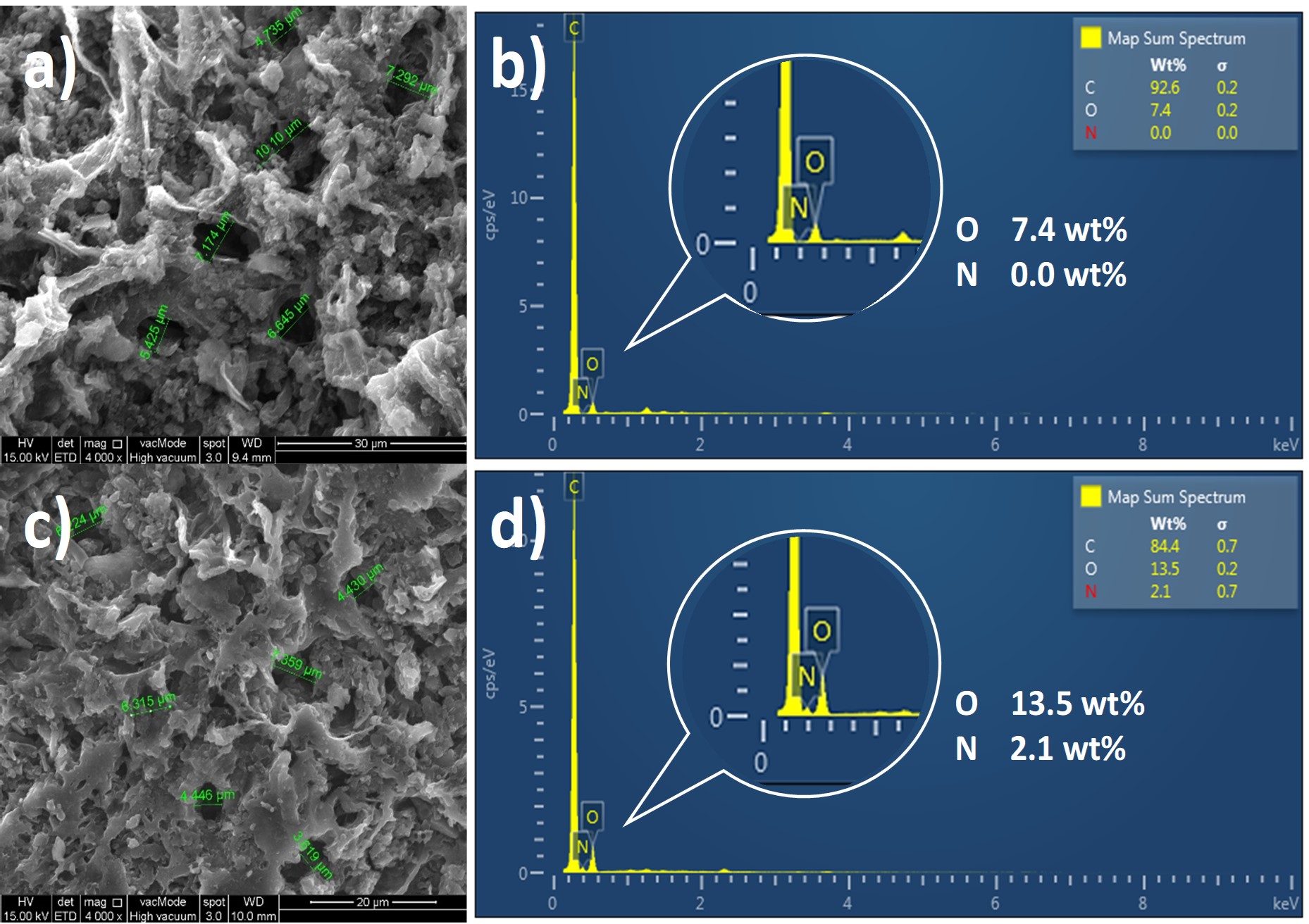
In this study egg white was used as a source of natural amino acids to modify the surface properties of palm shell activated carbon towards enhancing its CO2 capture performance. A simple impregnation method was employed for this purpose. Characterisation analysis was performed on the egg white-impregnated activated carbon to examine any changes on its surface properties prior to CO2 adsorption test. The modified adsorbent showed high thermal stability below 300°C and comprised of new amide functional group. Furthermore, the modified adsorbent exhibited 31% higher breakthrough time and maintained its CO2 adsorption capacity at 0.3 mmol/g in comparison to raw activated carbon, regardless of the reduction of surface area and micropore volume by 17% and 18% respectively. These findings provide evidence on the prospect of egg white-impregnated activated carbon for CO2 adsorption application which could pave the way for a new generation of affordable and eco-friendly adsorbents.
Downloads
References
Abdul Rani, N. H., & Muda, N. (2017). Impregnated Palm Kernel Shell Activated Carbon for CO2 Adsorption by Pressure Swing Adsorption. Indian Journal of Science and Technology, 10. https://doi.org/10.17485/ijst/2017/v10i2/110377
Allangawi, A., Alzaimoor, E. F. H., Shanaah, H. H., Mohammed, H. A., Saqer, H., El-Fattah, A. A., & Kamel, A. H. (2023). Carbon Capture Materials in Post-Combustion: Adsorption and Absorption-Based Processes. C, 9(1), 17. https://doi.org/10.3390/c9010017
Balsamo, M., Erto, A., Lancia, A., Totarella, G., Montagnaro, F., & Turco, R. (2018). Post-combustion CO2 capture: On the potentiality of amino acid ionic liquid as modifying agent of mesoporous solids. Fuel, 218, 155-161. https://doi.org/10.1016/j.fuel.2018.01.038
Chen, Y.-S., Ooi, C. W., Show, P. L., Hoe, B. C., Chai, W. S., Chiu, C.-Y., . . . Chang, Y.-K. (2022). Removal of Ionic Dyes by Nanofiber Membrane Functionalized with Chitosan and Egg White Proteins: Membrane Preparation and Adsorption Efficiency. Membranes, 12(1), 63. https://doi.org/10.3390/membranes12010063
U.S. EPA. (2023). Global Greenhouse Gas Emissions Data https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed August 28, 2023).
Gabelman, A. (2017). Adsorption Basics: Part 1. Chemical Engineering Progress. https://www.aiche.org/resources/publications/cep/2017/july/adsorption-basics-part-1 (accessed July 4, 2023)
Gholidoust, A., Atkinson, J. D., & Hashisho, Z. (2017). Enhancing CO2 Adsorption via Amine-Impregnated Activated Carbon from Oil Sands Coke. Energy & Fuels, 31(2), 1756-1763. https://doi.org/10.1021/acs.energyfuels.6b02800
Gil-Lalaguna, N., Navarro-Gil, Á., Carstensen, H.-H., Ruiz, J., Fonts, I., Ceamanos, J., . . . Gea, G. (2022). CO2 adsorption on pyrolysis char from protein-containing livestock waste: How do proteins affect? Science of The Total Environment, 846, 157395. https://doi.org/10.1016/j.scitotenv.2022.157395
Imtiaz-Ul-Islam, M., Hong, L., & Langrish, T. (2011). CO2 capture using whey protein isolate. Chemical Engineering Journal, 171(3), 1069-1081. https://doi.org/10.1016/j.cej.2011.05.003
Jahandar Lashaki, M., Khiavi, S., & Sayari, A. (2019). Stability of amine-functionalized CO2 adsorbents: a multifaceted puzzle. Chemical Society Reviews, 48(12), 3320-3405. https://doi.org/10.1039/C8CS00877A
Kaur, B., Gupta, R. K., & Bhunia, H. (2019). Chemically activated nanoporous carbon adsorbents from waste plastic for CO2 capture: Breakthrough adsorption study. Microporous and Mesoporous Materials, 282, 146-158. https://doi.org/10.1016/j.micromeso.2019.03.025
Ketabchi, M. R., Babamohammadi, S., Davies, W. G., Gorbounov, M., & Masoudi Soltani, S. (2023). Latest advances and challenges in carbon capture using bio-based sorbents: A state-of-the-art review. Carbon Capture Science & Technology, 6, 100087. https://doi.org/10.1016/j.ccst.2022.100087
Khan, U., Ogbaga, C. C., Abiodun, O.-A. O., Adeleke, A. A., Ikubanni, P. P., Okoye, P. U., & Okolie, J. A. (2023). Assessing absorption-based CO2 capture: Research progress and techno-economic assessment overview. Carbon Capture Science & Technology, 8, 100125. https://doi.org/10.1016/j.ccst.2023.100125
Le Quéré, C., Jackson, R. B., Jones, M. W., Smith, A. J. P., Abernethy, S., Andrew, R. M., . . . Peters, G. P. (2020). Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement. Nature Climate Change, 10(7), 647-653. https://doi.org/10.1038/s41558-020-0797-x
Ligero, A., Calero, M., Martín-Lara, M. Á., Blázquez, G., Solís, R. R., & Pérez, A. (2023). Fixed-bed CO2 adsorption onto activated char from the pyrolysis of a non-recyclable plastic mixture from real urban residues. Journal of CO2 Utilization, 73, 102517. https://doi.org/10.1016/j.jcou.2023.102517
Lu, Y.-R., Chen, H.-C., Liu, K., Liu, M., Chan, T.-S., & Hung, S.-F. (2022). Turn the Trash into Treasure: Egg-White-Derived Single-Atom Electrocatalysts Boost Oxygen Reduction Reaction. ACS Sustainable Chemistry & Engineering, 10(20), 6736-6742. https://doi.org/10.1021/acssuschemeng.2c00878
Mohamed Hatta, N. S., Hussin, F., Gew, L. T., & Aroua, M. K. (2023). Enhancing surface functionalization of activated carbon using amino acids from natural source for CO2 capture. Separation and Purification Technology, 313, 123468. https://doi.org/10.1016/j.seppur.2023.123468
Nazir, G., Rehman, A., Hussain, S., Mahmood, Q., Fteiti, M., Heo, K., . . . Aizaz Ud Din, M. (2023). Towards a sustainable conversion of biomass/biowaste to porous carbons for CO2 adsorption: recent advances, current challenges, and future directions. Green Chemistry, 25(13), 4941-4980. https://doi.org/10.1039/D3GC00636K
Parker, F. S. (1971). Amides and Amines. In F. S. Parker (Ed.), Applications of Infrared Spectroscopy in Biochemistry, Biology, and Medicine (pp. 165-172). Springer US. https://doi.org/10.1007/978-1-4684-1872-9_8
Philip, F. A., & Henni, A. (2023). Incorporation of Amino Acid-Functionalized Ionic Liquids into Highly Porous MOF-177 to Improve the Post-Combustion CO2 Capture Capacity. Molecules, 28(20), 7185. https://doi.org/10.3390/molecules28207185
Rasmussen, C. (2021). Emission Reductions From Pandemic Had Unexpected Effects on Atmosphere https://www.jpl.nasa.gov/news/emission-reductions-from-pandemic-had-unexpected-effects-on-atmosphere (accessed August 28, 2023)
Rugayah, A. F. A., A A; Norzita, N. (2014). Preparation and Characterisation of Activated Carbon from Palm Kernel Shell by Physical Activation with Steam. Journal of Oil Palm Research, 26(3), 251-264.
Saha, D., & Kienbaum, M. J. (2019). Role of oxygen, nitrogen and sulfur functionalities on the surface of nanoporous carbons in CO2 adsorption: A critical review. Microporous and Mesoporous Materials, 287, 29-55. https://doi.org/10.1016/j.micromeso.2019.05.051
Sashina, E. S., Golubikhin, A. Y., & Susanin, A. I. (2015). Prospects for Producing New Biomaterials Based on Fibroin. Fibre Chemistry, 47(4), 253-259. https://doi.org/10.1007/s10692-016-9675-8
Sharma, S., Kaur, M., Sharma, C., Choudhary, A., & Paul, S. (2021). Biomass-Derived Activated Carbon-Supported Copper Catalyst: An Efficient Heterogeneous Magnetic Catalyst for Base-Free Chan–Lam Coupling and Oxidations. ACS Omega, 6(30), 19529-19545. https://doi.org/10.1021/acsomega.1c01830
Shu Hui, T., & Ahmad Zaini, M. A. (2015). Potassium hydroxide activation of activated carbon: A commentary. Carbon Letters, 16, 275-280. https://doi.org/10.5714/CL.2015.16.4.275
Suhaili, N., Lim, L., Teh, L. P., Shahdan, S. N., & Manabu, Z. G. (2023). Effect of Arginine-Based Deep Eutectic Solvents on Supported Porous Sorbent for CO2. Sains Malaysiana, 52(5), 1419-1434. https://doi.org/10.17576/jsm-2023-5205-08
Vanda, H., Dai, Y., Wilson, E. G., Verpoorte, R., & Choi, Y. H. (2018). Green solvents from ionic liquids and deep eutectic solvents to natural deep eutectic solvents. Comptes Rendus Chimie, 21(6), 628-638. https://doi.org/10.1016/j.crci.2018.04.002
Wang, Q., Lei, L., Wang, F., Chen, C., Kang, X., Wang, C., . . . Chen, Z. (2020). Preparation of egg white@zeolitic imidazolate framework-8@polyacrylic acid aerogel and its adsorption properties for organic dyes. Journal of Solid State Chemistry, 292, 121656. https://doi.org/10.1016/j.jssc.2020.121656
You, C., & Kim, J. (2020). Quantitative risk assessment of an amine-based CO2 capture process. Korean Journal of Chemical Engineering, 37(10), 1649-1659. https://doi.org/10.1007/s11814-020-0567-5
Zhao, Y., Dong, Y., Guo, Y., Huo, F., Yan, F., & He, H. (2021). Recent progress of green sorbents-based technologies for low concentration CO2 capture. Chinese Journal of Chemical Engineering, 31, 113-125. https://doi.org/10.1016/j.cjche.2020.11.005
Zhu, Y., Fang, T., Hua, J., Qiu, S., Chu, H., Zou, Y., . . . Zeng, J.-L. (2019). Biomass-Derived Porous Carbon Prepared from Egg White for High-performance Supercapacitor Electrode Materials. ChemistrySelect, 4(24), 7358-7365. https://doi.org/10.1002/slct.201901632
Downloads
Published
How to Cite
Issue
Section
License
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).





