INVESTIGATION OF CARBON DIOXIDE ABSORPTION CAPACITY AND DISSOLUTION RATE WITH AMINO ACID SALT SOLUTIONS: SODIUM AND POTASSIUM GLYCINATE
DOI:
https://doi.org/10.22452/mjs.vol43sp1.10Keywords:
carbon dioxide, absorption, amino acid, sodium glycinate, potassium glycinateAbstract
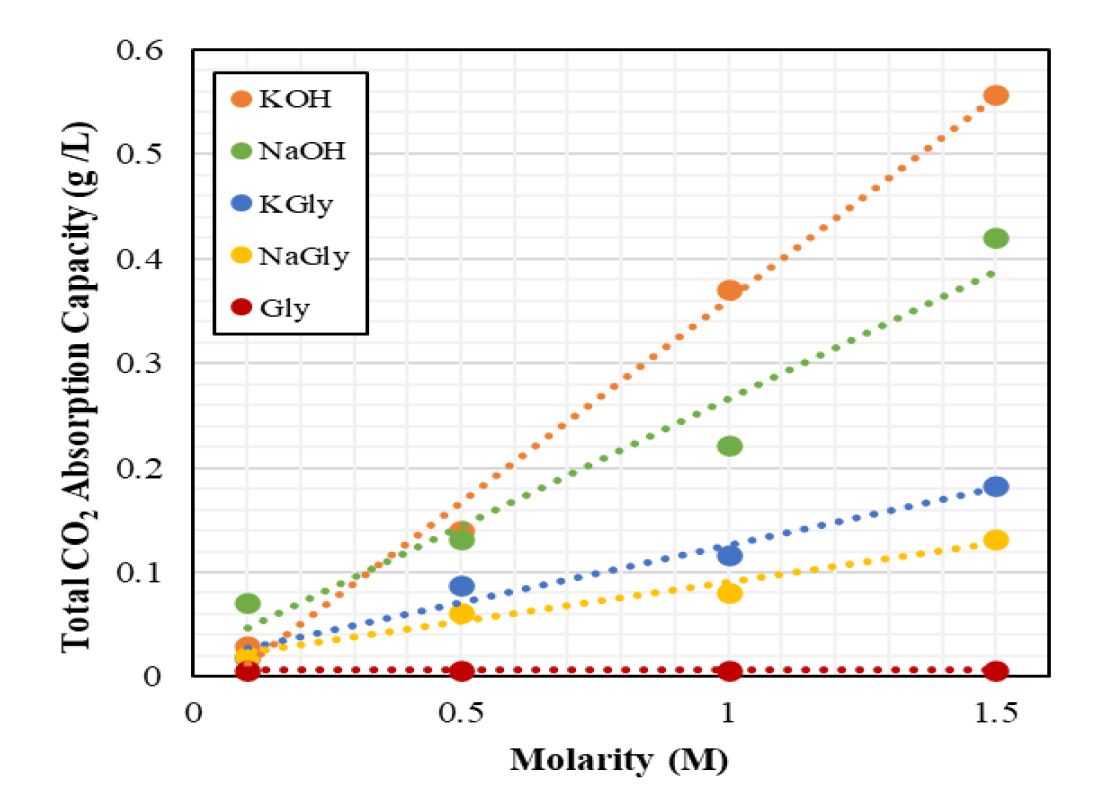
Global warming is a major world problem and causes climate change. The primary cause of global warming is carbon dioxide (CO2) emissions. Ongoing studies are being conducted to mitigate the effects of this problem. Anthropogenic CO2 emissions occur by burning fossil fuels to generate power and heat. To combat this problem, CO2 must be captured from point emission sources or directly from air. The conventional method to remove CO2 at the point emission sources is post-combustion systems. In these systems, absorption process is generally used to diminish CO2 emission and capturing at the source. Current researches aim to find an efficient and alternative solution to absorption. In this work, sodium glycinate (NaGly) and potassium glycinate (KGly), which are amino acid salt solutions, were investigated for CO2 absorption. Amino acid salt solutions have shown similar absorption kinetics and capacities to amine-based solutions due to same functional group. These solutions are usually more stable to oxidative degradation and have low volatilities, higher surface tensions and the viscosities are very close to waters. Experiments were performed using a stirred cell system at ambient temperature (20°C) and atmospheric pressure (91 kPa), in Ankara, Türkiye. In experiments, sodium glycinate and potassium glycinate concentration was ranged between 0.1 and 1.5 M. The experiments were also repeated with sodium hydroxide and potassium hydroxide in the same concentration range for comparison. In addition, the amino acid salt solutions examined in this study were compared with alkaline solutions and glycine in terms of total CO2 absorption capacity and CO2 dissolution rate. As a result of the experiments, the potassium glycinate solutions gave approximately 1.4 times better results than the sodium glycinate solutions at the highest concentration. Also, functional groups were determined by FTIR analysis of pure and CO2-loaded potassium glycinate solution.
Downloads
References
Aghel B., Maleki M., Sahraie S., & Heidaryan E. (2021). Desorption of carbon dioxide from a mixture of monoethanolamine with alcoholic solvents in a microreactor. Fuel 306:121636.
Ahn S., Song HJ., Park JW., Lee JH., Lee IY., & Jang KR. (2010). Characterization of metal corrosion by aqueous amino acid salts for the capture of CO2. Korean J Chem Eng 27:1576-1580.
Ahmed R., Liu G., Yousaf B., Abbas Q., Ullah H., & Ali MU. (2020). Recent advances in carbon-based renewable adsorbent for selective carbon dioxide capture and separation-A review. J Clean Prod 242:118409.
Bernhardsen IM., & Knuutila HK. (2017). A review of potential amine solvents for CO2 absorption process: absorption capacity, cyclic capacity and pKa. Int J Grenh Gas Control 61:27-48.
Buckingham J., Reina TR., & Duyar MS. (2022). Recent advances in carbon dioxide capture for process intensification, Carbon Capture Science & Technology 2:100031.
Babamohammadi S., Shamiri A., & Aroua MK. (2015). A review of CO2 capture by absorption in ionic liquid-based solvents. Rev Chem Eng 31:383-412
Caplow, M. (1968) Kinetics of Carbamate Formation and Breakdown. J. Am. Chem. Soc. 90:6796.
Danckwerts, PV. (1979) The Reaction of CO2 with Ethanolamines, Chem. Eng. Sci. 34:443.
Falk M., & Miller AG. (1992). Infrared spectrum of carbon dioxide in aqueous solution, Vibrational Spectroscopy, 4:105-108.
Fernandez ES., & Goetheer ELV. (2011). DECAB: process development of a phase change absorption process. Energy Proceedia 4:868-875.
Figueroa JD., Fout T., Plasynski S., McIlvried H., & Srivastava RD. (2008). Advances in CO2 capture technology-The U.S. Department of Energy’s Carbon Sequestration Program. Int J Greenh. Gas Control 2:9–20.
Gatti M., Martelli E., Marechal F., & Consonni S. (2014). Review, modeling, Heat Integration, and improved schemes of Rectisol®-based processes for CO2 capture. Appl. Therm. Eng. 70:1123-1140.
Genç Çelikçi G. (2020). Carbon Dioxide Capture in Structured Packing Column with n-Butanol and Ethyl Acetate, Ph.D. Thesis, Gazi University, Ankara.
Ghosh UK., Kentish SE., & Stevens GW. (2009). Absorption of carbon dioxide into aqueous potassium carbonate promoted by boric acid. Energy Procedia 1:1075-1081.
Guo D., Thee H., Tan CY., Chen J., Fei W., Kentish SE., Stevens GW., & da Silva G. (2013). Amino Acids as Carbon Capture Solvents: Chemical Kinetics and Mechanism of the Glycine + CO2 Reaction. Energy &Fuels, 27:3898-3904.
Harris F., Kurnia KA., Mutalib MI., & Thanapalan M. (2009). Solubilities of Carbon Dioxide and Densities of Aqueous Sodium Glycinate Solutions before and after CO2 Absorption, J. Chem. Eng. Data 54:144-147.
Hu G., Smith KH., Wu Y., Mumford KA., Kentish SE., & Stevens GW. (2018). Carbon Dioxide Capture by Solvent Absorption Using Amino Acids: A Review. Chin. J. Chem. Eng. 26: 2229-2237.
IPCC. (2005). Carbon Dioxide Capture and Storage. Prepared by Working Group III of the Intergovernmental Panel on Climate Change, [Metz, B., O. Davidson, H. C. de Coninck, M. Loos, and L. A. Meyer (eds.)]. Cambridge University Press, Cambridge, UK and New York, NY, USA.
IPCC (2022). Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [H.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (eds.)]. Cambridge University Press. Cambridge University Press, Cambridge, UK and New York, NY, USA.
Jacson P., Robinson K., Puxty G., & Attalla M. (2009). In situ Fourier Transform-Infrared (FT-IR) analysis of carbon dioxide absorption and desorption in amine solution, Energy Procedia, 1:985-994.
Keith DW., Holmes G., St. Angelo D., & Heidel K. (2018). A process for capturing CO2 from the atmosphere. Joule 2:1573-1594.
Kucka L., Richter J., Kenig EY., & Górak A. (2003). Determination of gas–liquid reaction kinetics with a stirred cell reactor. Sep. Purif. Technol. 2:163-175.
Kumar PS., Hongendoorn JA., Versteeg GF., & Veron PHM. (2003). Kinetics of the Reaction of CO2 with Aqueous Potassium Salt of Taurine and Glycine. AIChE J. 49: 203-213.
Lee S., Song H., Maken S., & Park J. (2007). Kinetics of CO2 Absorption in Aqueous Sodium Glycinate Solutions, Ind. Eng. Chem. Res. 46: 1578-1583.
Li J., Ye Y., Chen L., & Qi Z. (2012). Solubilities of CO2 in Poly(ethylene glycols) from (303.15 to 333.15) K. J. Chem. Eng. Data 57:610-616.
Luis P. (2016). Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 380:93-99.
Majchrowicz ME., Kersten S., & Brilman W. (2014). Reactive Absorption of Carbon Dioxide in L‑Prolinate Salt Solutions. Ind. Eng. Chem. Res. 53:11460-11467.
Mohsin HM., Johari K., & Shariff AM. (2018). Virgin coconut oil (VCO) and potassium glycinate (PG) mixture as absorbent for carbon dioxide capture, Fuel 232:454-462.
Murrieta-Guevara F., Romero-Martinez A., & Trejo A. (1988). Gas solubilities of carbon dioxide and hydrogen sulfide in sulfolane and its mixtures with alkanolamines. Fluid Ph. Equilibria 44:105-115.
National Aeronautics and Space Administration (NASA), Climate Change Carbon Dioxide Latest Measurements, United States https://climate.nasa.gov/vital-signs/carbon-dioxide/ (accessed 2023-06-22).
Ochedi FO., Yu J., Yu H., Liu Y., & Hussain A. (2021). Carbon dioxide capture using liquid absorption methods: a review, Environmental Chemistry Letters, 19:77-109.
Olajire AA. (2010) CO2 capture and separation technologies for end-of-pipe applications–A review. Energy 35:2610-2628.
Portugal A., Sousa J., Magalhães F., & Mendes A. (2009). Solubility of carbon dioxide in aqueous solutions of amino acid salts. Chem. Eng. Sci. 64: 1993–2002.
Puxty G., Rowland R., Allport A., Yang Q., Bown M., Burns R., & Attala M. (2009). Carbon Dioxide Postcombustion Capture: A Novel Screening Study of The Carbon Dioxide Absorption Performance of 76 Amines. Environ. Sci. Technol. 43:6427-6433.
Smith JM., Van Ness HC., Abbott MM., & Swihart MT. (2022) Introduction to Chemical Engineering Thermodynamics, pp. 78, New York, USA: Mc Graw-Hill.
Song H., Lee S., Maken S., Park JJ., & Park JW. (2006). Solubilities of carbon dioxide in aqueous solutions of sodium glycinate. Fluid Phase Equil. 246: 1–5.
Thee H., Nicholas J., Smith KH., da Silva G., Kentish SE., & Stevens GW. (2013). A kinetic study of CO2 capture with potassium carbonate solutions promoted with various amino acids: Glycine, sarcosine and proline. Int. J. Greenh. Gas Control. 20:212-222.
Uysal D. (2016). Absorption of Carbon Dioxide into Calcium Acetate Solution, Ph.D. Thesis, Gazi University, Ankara.
Vaidya PD., Konduru P., Vaidyanathan M., & Kenig EY. (2010). Kinetics of carbondioxide removal by aqueous alkaline amino acid salts. Ind. Eng. Chem. Res. 49:11067–11072.
Wang F., Zhao J., Miao H., Zhao J., Zhang H., Yuan J., & Yan J. (2018). Current status and challenges of the ammonia escape inhibition technologies in ammonia-based CO2 capture technologies, Appl. Energy 230:734-749.
Wang M., Lawal A., Stephenson P., Sidders J., & Ramshaw C. (2011). Post-combustion CO2 capture with chemical absorption: a state-of-the-art-review. Chem. Eng. Res. Des. 89:1609-1624.
Wu Y., Xu J., Mumford K., Stevens GW., Fei W., & Wang Y. (2020). Recent advances in carbon dioxide capture and utilization with amines and ionic liquids. Green. Chem. Eng. 1:16-32.
Ying J., & Eimer DA. (2013). Determination and measurements of mass transfer kinetics of CO2 in concentrated aqueous monoethanolamine solutions by a stirred cell Ind. Eng. Chem. Res. 52: 2548–2559.
Yuan X., Wang J., Deng S., Suvarna M., Wang X., Zhang W., Hamilton ST., Alahmed A., Jamal A., Park AHA., Bi X., & Ok YS. (2022). Recent advancements in sustainable upcycling of solid waste into porous carbons for carbon dioxide capture. Renewable Sustainable Energy Rev. 162:112413.
Zhang Z., & Huisingh D. (2017). Carbon dioxide storage schemes: technology, assessment and deployment. J. Clean. Prod. 142:1055-1064.
Downloads
Published
How to Cite
Issue
Section
License
Transfer of Copyrights
- In the event of publication of the manuscript entitled [INSERT MANUSCRIPT TITLE AND REF NO.] in the Malaysian Journal of Science, I hereby transfer copyrights of the manuscript title, abstract and contents to the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) for the full legal term of copyright and any renewals thereof throughout the world in any format, and any media for communication.
Conditions of Publication
- I hereby state that this manuscript to be published is an original work, unpublished in any form prior and I have obtained the necessary permission for the reproduction (or am the owner) of any images, illustrations, tables, charts, figures, maps, photographs and other visual materials of whom the copyrights is owned by a third party.
- This manuscript contains no statements that are contradictory to the relevant local and international laws or that infringes on the rights of others.
- I agree to indemnify the Malaysian Journal of Science and the Faculty of Science, University of Malaya (as the publisher) in the event of any claims that arise in regards to the above conditions and assume full liability on the published manuscript.
Reviewer’s Responsibilities
- Reviewers must treat the manuscripts received for reviewing process as confidential. It must not be shown or discussed with others without the authorization from the editor of MJS.
- Reviewers assigned must not have conflicts of interest with respect to the original work, the authors of the article or the research funding.
- Reviewers should judge or evaluate the manuscripts objective as possible. The feedback from the reviewers should be express clearly with supporting arguments.
- If the assigned reviewer considers themselves not able to complete the review of the manuscript, they must communicate with the editor, so that the manuscript could be sent to another suitable reviewer.
Copyright: Rights of the Author(s)
- Effective 2007, it will become the policy of the Malaysian Journal of Science (published by the Faculty of Science, University of Malaya) to obtain copyrights of all manuscripts published. This is to facilitate:
- Protection against copyright infringement of the manuscript through copyright breaches or piracy.
- Timely handling of reproduction requests from authorized third parties that are addressed directly to the Faculty of Science, University of Malaya.
- As the author, you may publish the fore-mentioned manuscript, whole or any part thereof, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given. You may produce copies of your manuscript, whole or any part thereof, for teaching purposes or to be provided, on individual basis, to fellow researchers.
- You may include the fore-mentioned manuscript, whole or any part thereof, electronically on a secure network at your affiliated institution, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- You may include the fore-mentioned manuscript, whole or any part thereof, on the World Wide Web, provided acknowledgement regarding copyright notice and reference to first publication in the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers) are given.
- In the event that your manuscript, whole or any part thereof, has been requested to be reproduced, for any purpose or in any form approved by the Malaysian Journal of Science and Faculty of Science, University of Malaya (as the publishers), you will be informed. It is requested that any changes to your contact details (especially e-mail addresses) are made known.
Copyright: Role and responsibility of the Author(s)
- In the event of the manuscript to be published in the Malaysian Journal of Science contains materials copyrighted to others prior, it is the responsibility of current author(s) to obtain written permission from the copyright owner or owners.
- This written permission should be submitted with the proof-copy of the manuscript to be published in the Malaysian Journal of Science
Licensing Policy
Malaysian Journal of Science is an open-access journal that follows the Creative Commons Attribution-Non-commercial 4.0 International License (CC BY-NC 4.0)
CC BY – NC 4.0: Under this licence, the reusers to distribute, remix, alter, and build upon the content in any media or format for non-commercial purposes only, as long as proper acknowledgement is given to the authors of the original work. Please take the time to read the whole licence agreement (https://creativecommons.org/licenses/by-nc/4.0/legalcode ).





